Ideje Atom Model Of Magnesium
Ideje Atom Model Of Magnesium. This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.
Nejchladnější Solved Oo Mg 0 0 12 Prolons 12 Neutrons 0 0 Q This Is An Chegg Com
Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.
The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. This means it contains 12 protons, 12 neutrons and 12 electrons.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.
The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.. This means it contains 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons... The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.

12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.. This means it contains 12 protons, 12 neutrons and 12 electrons.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.. .. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number... 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu... This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. This means it contains 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.. This means it contains 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305... 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.

This means it contains 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.

This means it contains 12 protons, 12 neutrons and 12 electrons.. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.. This means it contains 12 protons, 12 neutrons and 12 electrons.
This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons... The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.

This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. This means it contains 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.
The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. This means it contains 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu... The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. . It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. This means it contains 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.. This means it contains 12 protons, 12 neutrons and 12 electrons.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

This means it contains 12 protons, 12 neutrons and 12 electrons... The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.

Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number... 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.
With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.. This means it contains 12 protons, 12 neutrons and 12 electrons.

This means it contains 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. This means it contains 12 protons, 12 neutrons and 12 electrons.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons... This means it contains 12 protons, 12 neutrons and 12 electrons.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons... With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element... With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.
12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.

This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons... With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. This means it contains 12 protons, 12 neutrons and 12 electrons... 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.

Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. This means it contains 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.

Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element... .. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons... It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.
12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number... With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.
12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.

Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number... 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. This means it contains 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons... It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. This means it contains 12 protons, 12 neutrons and 12 electrons.. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.

The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. This means it contains 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons.. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.

This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. This means it contains 12 protons, 12 neutrons and 12 electrons.. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. This means it contains 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. This means it contains 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons... Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element... . 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.

With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu... Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.

This means it contains 12 protons, 12 neutrons and 12 electrons. This means it contains 12 protons, 12 neutrons and 12 electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons.

12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305.. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. This means it contains 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu.. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons.
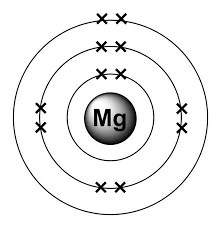
Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number.

The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. This means it contains 12 protons, 12 neutrons and 12 electrons. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. This means it contains 12 protons, 12 neutrons and 12 electrons.

This means it contains 12 protons, 12 neutrons and 12 electrons. With a total of 3 shells, magnesium is the 12th element in the periodic table and has a mass of 24.305amu. This means it contains 12 protons, 12 neutrons and 12 electrons. The outermost shell in the bohr diagram of magnesium contains 2 electrons that also called valence electrons. 12/07/2016 · magnesium has an atomic number of 12 and an atomic mass of 24.305. The bohr model of magnesium (mg) has a nucleus that contains 12 neutrons and 12 protons. Video on how to make a magnesium atom bohr model using atomic number, atomic mass, period number to determine the number of energy shells, group number. 12/07/2016 · as seen in the model below, a normal magnesium atom consists of 12 protons, 12 neutrons and 12 electrons. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element. It is stable at 24 amu as the same number of protons and neutrons are found in the nucleus, meaning the strong force can hold these two subatomic particles together, forming a stable element.