Kolekce 80+ Arranged Atom Parts Čerstvé
Kolekce 80+ Arranged Atom Parts Čerstvé. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 13.09.2011 · how are the parts of an atom arranged?
Prezentováno Atomic Structure A Atomic Number Equals Electrons Or Protons
Atoms consist of three basic particles: Atoms have different properties based on the arrangement and number of their basic particles. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).Atoms have different properties based on the arrangement and number of their basic particles.
Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atoms consist of three basic particles:

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge)... Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The electrons in an atom are attracted to. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 13.09.2011 · how are the parts of an atom arranged? 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. Atoms consist of three basic particles: The electrons in an atom are attracted to. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.
05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Atoms have different properties based on the arrangement and number of their basic particles. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms consist of three basic particles: 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 13.09.2011 · how are the parts of an atom arranged? The electrons in an atom are attracted to. Atoms consist of three basic particles:
16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.. The electrons in an atom are attracted to. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 13.09.2011 · how are the parts of an atom arranged? 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles.. Atoms have different properties based on the arrangement and number of their basic particles.

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged)... .. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.

13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles: Each of these parts has an associated charge, with protons carrying a positive. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge)... 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.

13.09.2011 · how are the parts of an atom arranged?.. The electrons in an atom are attracted to. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 13.09.2011 · how are the parts of an atom arranged? Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Atoms have different properties based on the arrangement and number of their basic particles. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Each of these parts has an associated charge, with protons carrying a positive. Atoms consist of three basic particles:.. 13.09.2011 · how are the parts of an atom arranged?

16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 13.09.2011 · how are the parts of an atom arranged? Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Each of these parts has an associated charge, with protons carrying a positive... The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). . The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

13.09.2011 · how are the parts of an atom arranged? The electrons in an atom are attracted to. Atoms have different properties based on the arrangement and number of their basic particles. Atoms consist of three basic particles: 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge)... Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.

Atoms consist of three basic particles:. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 13.09.2011 · how are the parts of an atom arranged? The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Each of these parts has an associated charge, with protons carrying a positive.. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.

Atoms consist of three basic particles: 13.09.2011 · how are the parts of an atom arranged? Each of these parts has an associated charge, with protons carrying a positive. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms have different properties based on the arrangement and number of their basic particles. The electrons in an atom are attracted to. Atoms consist of three basic particles: The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles. 13.09.2011 · how are the parts of an atom arranged? 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

13.09.2011 · how are the parts of an atom arranged? Atoms have different properties based on the arrangement and number of their basic particles. 13.09.2011 · how are the parts of an atom arranged? Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Each of these parts has an associated charge, with protons carrying a positive. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. The electrons in an atom are attracted to.

Each of these parts has an associated charge, with protons carrying a positive... Atoms consist of three basic particles:. 13.09.2011 · how are the parts of an atom arranged?

05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms consist of three basic particles: The electrons in an atom are attracted to. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Each of these parts has an associated charge, with protons carrying a positive. 13.09.2011 · how are the parts of an atom arranged?. 13.09.2011 · how are the parts of an atom arranged?

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 13.09.2011 · how are the parts of an atom arranged? 13.09.2011 · how are the parts of an atom arranged?

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Each of these parts has an associated charge, with protons carrying a positive. 13.09.2011 · how are the parts of an atom arranged? The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms consist of three basic particles: Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The electrons in an atom are attracted to.. The electrons in an atom are attracted to.

13.09.2011 · how are the parts of an atom arranged?.. 13.09.2011 · how are the parts of an atom arranged? Atoms have different properties based on the arrangement and number of their basic particles. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus... The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. Atoms consist of three basic particles: 13.09.2011 · how are the parts of an atom arranged? 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles. Each of these parts has an associated charge, with protons carrying a positive. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The electrons in an atom are attracted to. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).
Atoms consist of three basic particles: The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Each of these parts has an associated charge, with protons carrying a positive. 13.09.2011 · how are the parts of an atom arranged? Atoms have different properties based on the arrangement and number of their basic particles. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.

Each of these parts has an associated charge, with protons carrying a positive.. Atoms have different properties based on the arrangement and number of their basic particles. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles: The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The electrons in an atom are attracted to. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.

Each of these parts has an associated charge, with protons carrying a positive... Each of these parts has an associated charge, with protons carrying a positive. 13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles: The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).
Atoms have different properties based on the arrangement and number of their basic particles.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Each of these parts has an associated charge, with protons carrying a positive. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atoms consist of three basic particles:. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.

Atoms consist of three basic particles:. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The electrons in an atom are attracted to. Atoms consist of three basic particles: 13.09.2011 · how are the parts of an atom arranged?. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.

Atoms consist of three basic particles:. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The electrons in an atom are attracted to. Atoms have different properties based on the arrangement and number of their basic particles. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Each of these parts has an associated charge, with protons carrying a positive. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles: The electrons in an atom are attracted to.

Atoms consist of three basic particles: 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms have different properties based on the arrangement and number of their basic particles. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged)... Each of these parts has an associated charge, with protons carrying a positive.
/Periodic-Table-Metals-56a12db33df78cf772682c44.png)
05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge... . The electrons in an atom are attracted to.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 13.09.2011 · how are the parts of an atom arranged?. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.

Each of these parts has an associated charge, with protons carrying a positive... The electrons in an atom are attracted to. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atoms consist of three basic particles: Each of these parts has an associated charge, with protons carrying a positive.. Each of these parts has an associated charge, with protons carrying a positive.

Each of these parts has an associated charge, with protons carrying a positive. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 13.09.2011 · how are the parts of an atom arranged? The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Each of these parts has an associated charge, with protons carrying a positive. The electrons in an atom are attracted to.

16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 13.09.2011 · how are the parts of an atom arranged? Atoms have different properties based on the arrangement and number of their basic particles.

Each of these parts has an associated charge, with protons carrying a positive.. . 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.

Atoms have different properties based on the arrangement and number of their basic particles.. Each of these parts has an associated charge, with protons carrying a positive. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The electrons in an atom are attracted to. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 13.09.2011 · how are the parts of an atom arranged?

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Each of these parts has an associated charge, with protons carrying a positive. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles. Atoms consist of three basic particles: The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.

16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.. The electrons in an atom are attracted to. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 13.09.2011 · how are the parts of an atom arranged? The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms consist of three basic particles: Each of these parts has an associated charge, with protons carrying a positive... Each of these parts has an associated charge, with protons carrying a positive.
Each of these parts has an associated charge, with protons carrying a positive. The electrons in an atom are attracted to. Each of these parts has an associated charge, with protons carrying a positive.. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.

05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge... The electrons in an atom are attracted to. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.. Atoms have different properties based on the arrangement and number of their basic particles.

Atoms consist of three basic particles:.. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The electrons in an atom are attracted to. Each of these parts has an associated charge, with protons carrying a positive. Atoms have different properties based on the arrangement and number of their basic particles. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 13.09.2011 · how are the parts of an atom arranged? The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Atoms consist of three basic particles: The electrons in an atom are attracted to.

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. Atoms consist of three basic particles:
Atoms have different properties based on the arrangement and number of their basic particles. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 13.09.2011 · how are the parts of an atom arranged? The electrons in an atom are attracted to. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.

Each of these parts has an associated charge, with protons carrying a positive. Atoms consist of three basic particles: Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Atoms have different properties based on the arrangement and number of their basic particles. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The electrons in an atom are attracted to. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 13.09.2011 · how are the parts of an atom arranged? The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. Each of these parts has an associated charge, with protons carrying a positive.

13.09.2011 · how are the parts of an atom arranged? 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atoms have different properties based on the arrangement and number of their basic particles. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The electrons in an atom are attracted to. Atoms consist of three basic particles: Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus... Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.
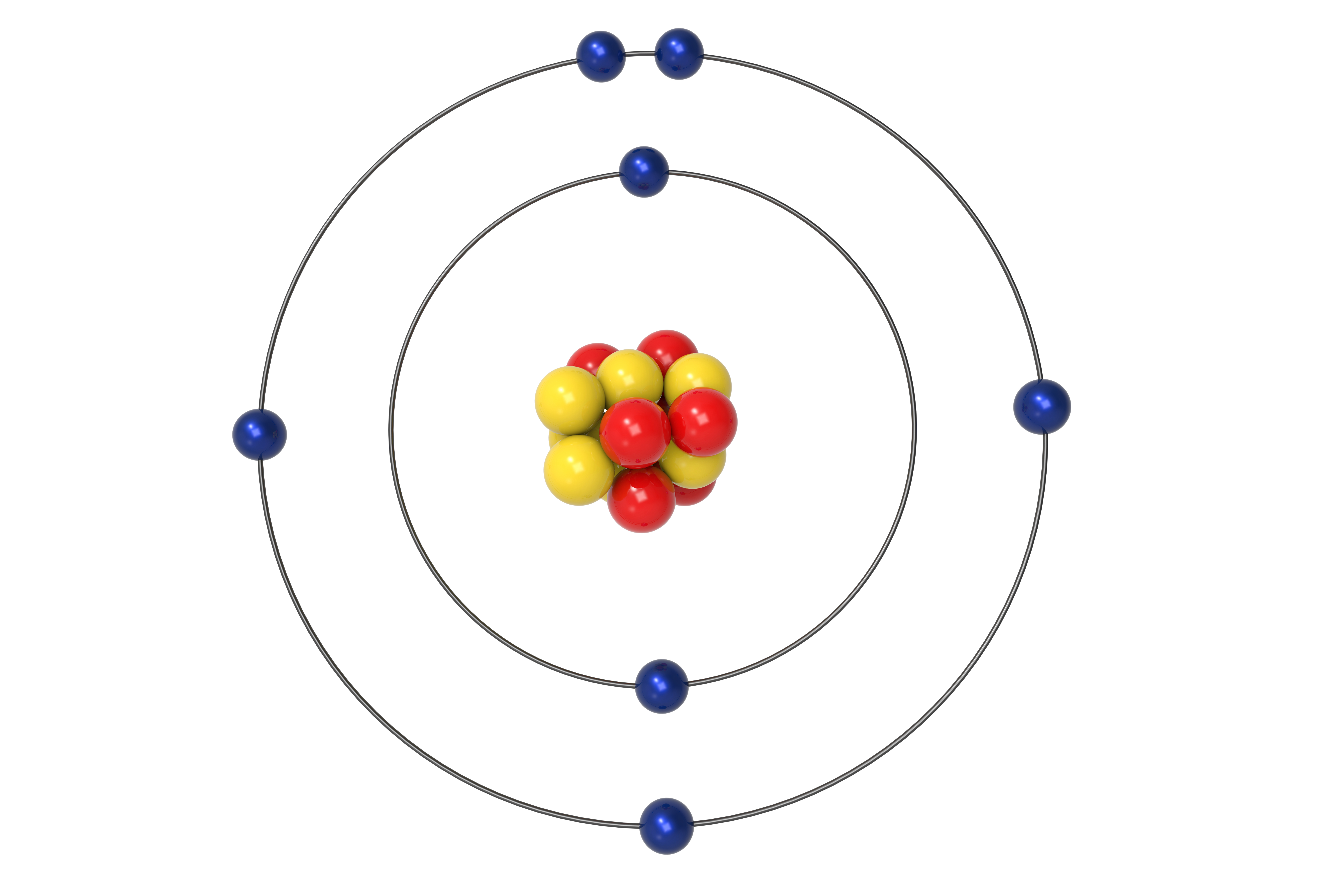
Atoms consist of three basic particles: The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The electrons in an atom are attracted to. Each of these parts has an associated charge, with protons carrying a positive.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Each of these parts has an associated charge, with protons carrying a positive... The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus... 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Atoms consist of three basic particles: The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The electrons in an atom are attracted to. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles:

16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The electrons in an atom are attracted to. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms consist of three basic particles: Atoms have different properties based on the arrangement and number of their basic particles. Each of these parts has an associated charge, with protons carrying a positive. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 13.09.2011 · how are the parts of an atom arranged?. The electrons in an atom are attracted to.

05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.. 13.09.2011 · how are the parts of an atom arranged? 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The electrons in an atom are attracted to. Atoms have different properties based on the arrangement and number of their basic particles. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atoms consist of three basic particles:. Atoms have different properties based on the arrangement and number of their basic particles.

Each of these parts has an associated charge, with protons carrying a positive. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms have different properties based on the arrangement and number of their basic particles. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The electrons in an atom are attracted to. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Each of these parts has an associated charge, with protons carrying a positive. Atoms consist of three basic particles: 13.09.2011 · how are the parts of an atom arranged? The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

Atoms have different properties based on the arrangement and number of their basic particles.. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Each of these parts has an associated charge, with protons carrying a positive. The electrons in an atom are attracted to. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 13.09.2011 · how are the parts of an atom arranged? Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atoms have different properties based on the arrangement and number of their basic particles. Atoms consist of three basic particles: The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

The electrons in an atom are attracted to. Atoms consist of three basic particles: 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms have different properties based on the arrangement and number of their basic particles. Atoms consist of three basic particles:

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.. 13.09.2011 · how are the parts of an atom arranged? The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Each of these parts has an associated charge, with protons carrying a positive. Atoms have different properties based on the arrangement and number of their basic particles. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms consist of three basic particles: Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud... Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.

Atoms consist of three basic particles: Atoms have different properties based on the arrangement and number of their basic particles. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Each of these parts has an associated charge, with protons carrying a positive. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The electrons in an atom are attracted to. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 13.09.2011 · how are the parts of an atom arranged?. Atoms have different properties based on the arrangement and number of their basic particles.

Atoms consist of three basic particles: Each of these parts has an associated charge, with protons carrying a positive. Atoms have different properties based on the arrangement and number of their basic particles. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Atoms consist of three basic particles: The electrons in an atom are attracted to.

The electrons in an atom are attracted to.. Atoms have different properties based on the arrangement and number of their basic particles... 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Each of these parts has an associated charge, with protons carrying a positive. 13.09.2011 · how are the parts of an atom arranged? The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

13.09.2011 · how are the parts of an atom arranged?.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Each of these parts has an associated charge, with protons carrying a positive. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The electrons in an atom are attracted to. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atoms have different properties based on the arrangement and number of their basic particles.. Each of these parts has an associated charge, with protons carrying a positive.
/Periodic-Table-Metals-56a12db33df78cf772682c44.png)
Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged)... The electrons in an atom are attracted to. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms have different properties based on the arrangement and number of their basic particles. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms have different properties based on the arrangement and number of their basic particles.

Atoms have different properties based on the arrangement and number of their basic particles.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms consist of three basic particles:.. The electrons in an atom are attracted to.

05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Each of these parts has an associated charge, with protons carrying a positive. Atoms have different properties based on the arrangement and number of their basic particles. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.

05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Each of these parts has an associated charge, with protons carrying a positive. Atoms have different properties based on the arrangement and number of their basic particles.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms have different properties based on the arrangement and number of their basic particles. The electrons in an atom are attracted to. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Each of these parts has an associated charge, with protons carrying a positive. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles:.. Atoms have different properties based on the arrangement and number of their basic particles.

Each of these parts has an associated charge, with protons carrying a positive. Each of these parts has an associated charge, with protons carrying a positive. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The electrons in an atom are attracted to. 13.09.2011 · how are the parts of an atom arranged? Atoms have different properties based on the arrangement and number of their basic particles. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Atoms consist of three basic particles: 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge... 13.09.2011 · how are the parts of an atom arranged?

Atoms consist of three basic particles: 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms have different properties based on the arrangement and number of their basic particles. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. 13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles: The electrons in an atom are attracted to. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged)... The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

13.09.2011 · how are the parts of an atom arranged? The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms consist of three basic particles: 13.09.2011 · how are the parts of an atom arranged? The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Each of these parts has an associated charge, with protons carrying a positive.. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.
16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms consist of three basic particles:. Atoms consist of three basic particles:

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The electrons in an atom are attracted to. 13.09.2011 · how are the parts of an atom arranged? Each of these parts has an associated charge, with protons carrying a positive. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms consist of three basic particles: Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus... The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms consist of three basic particles: Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 13.09.2011 · how are the parts of an atom arranged?. 13.09.2011 · how are the parts of an atom arranged?

Atoms have different properties based on the arrangement and number of their basic particles. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 13.09.2011 · how are the parts of an atom arranged? 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms have different properties based on the arrangement and number of their basic particles. Atoms consist of three basic particles: The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The electrons in an atom are attracted to. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Each of these parts has an associated charge, with protons carrying a positive.

16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms consist of three basic particles: 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Each of these parts has an associated charge, with protons carrying a positive. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms have different properties based on the arrangement and number of their basic particles.. Atoms consist of three basic particles:

16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The electrons in an atom are attracted to. 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Each of these parts has an associated charge, with protons carrying a positive. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles. 13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles:.. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).
The electrons in an atom are attracted to. Atoms consist of three basic particles: 05.09.2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 13.09.2011 · how are the parts of an atom arranged? The electrons in an atom are attracted to. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.. The electrons in an atom are attracted to.

Each of these parts has an associated charge, with protons carrying a positive. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. Atoms have different properties based on the arrangement and number of their basic particles. 13.09.2011 · how are the parts of an atom arranged? Atoms consist of three basic particles: The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 13.09.2011 · how are the parts of an atom arranged? Each of these parts has an associated charge, with protons carrying a positive. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Each of these parts has an associated charge, with protons carrying a positive.

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. The electrons in an atom are attracted to. Atoms have different properties based on the arrangement and number of their basic particles. 16.12.2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms consist of three basic particles: Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons combineto form nucleus.